 The sunsets here have been quite lovely the last few days, and I suspected that the ash and sulfur dioxide plume from the recent eruptions at Sarychev were responsible. SpaceWeather has confirmed that with a nice photo gallery of colorful sunsets. SpaceWeather also posted a set of photos of the eruption as captured from the ISS that allow sterescopic viewing either with a stereoscope or cross-eyed. Or, if you prefer, APOD posted a red-green glasses version a few days ago.
The sunsets here have been quite lovely the last few days, and I suspected that the ash and sulfur dioxide plume from the recent eruptions at Sarychev were responsible. SpaceWeather has confirmed that with a nice photo gallery of colorful sunsets. SpaceWeather also posted a set of photos of the eruption as captured from the ISS that allow sterescopic viewing either with a stereoscope or cross-eyed. Or, if you prefer, APOD posted a red-green glasses version a few days ago.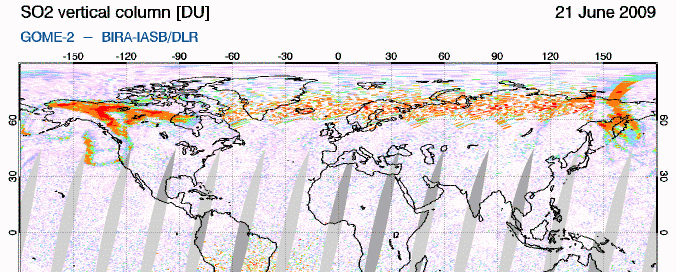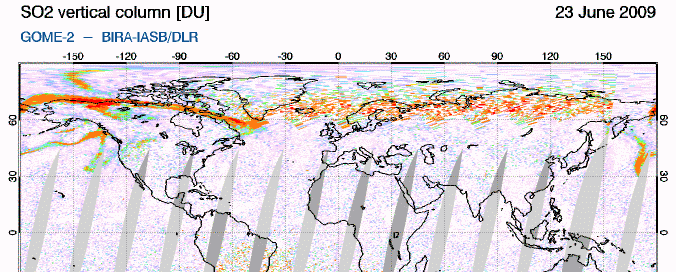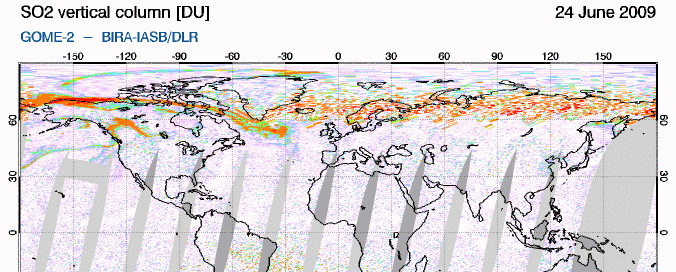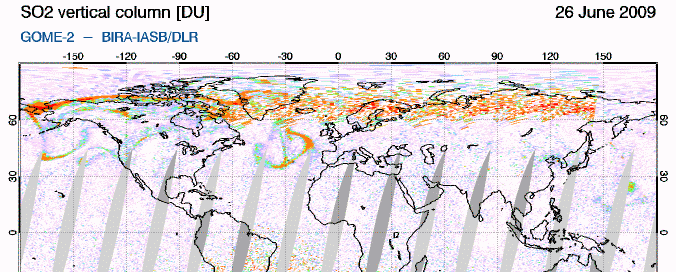
GIF animation of satellite data showing the movement of Sarychev SO2 (orange) from June 21-26So how does this work? SO2 oxidizes to sulfate, SO4, in the atmosphere. The sulfate, in turn, forms very small haze particles. Tiny particles of either solid sulfate or liquid sulfuric acid in the stratosphere are very effective at scattering out the red light component of (white) light coming from the sun. The light not scattered out is thus left "bluer" or "more violet" than would ususally be the case. Consider the standard ROYGBIV representation of the spectrum... if you scatter out more of the longer wavelengths (the red end), you're left with a proportionally intensified violet end: roygBIV. So during volcanic sunsets, reds and oranges are intensified near the horizon, blues in the mid sky, and purple-violet overhead. (see the Wikipedia "sunset colors" for more info)
Incidentally, if the sulfate is in the lower, mixing portion of the atmosphere (the troposphere, from ground level to ~10+ km), it just makes haze. The sulfate needs to be in the next level up, the stratosphere, to get the great color. This is why sunsets in areas that rely on coal-burning have pretty bland colors. The smokestacks don't get the resulting SO2 up high enough.
My limited perambulations don't really get me to open areas where I can see sunsets uncluttered with buildings and trees, but it might be worth my while to walk over to the west end of campus this evening to see what I can see.
My limited perambulations don't really get me to open areas where I can see sunsets uncluttered with buildings and trees, but it might be worth my while to walk over to the west end of campus this evening to see what I can see.










No comments:
Post a Comment